Overview
The following is an overview of the CDISC ODM, SDTM and DEFINE.XML
training performed at MXI.When
Part 1 - Wednesday, August 12, 2009 9:00 AM-4:00 PM PST
Part 2 - Thursday, August 13, 2009 9:00 AM-4:00 PM PST
Where
MXI Training Room
2185 Oakland Rd
San Jose, CA 95131
Course Schedule
| Date /
Time
|
Description
|
Wednesday,
August 12
9:00 am - 12:00 noon |
CDISC ODM Implementation Operational Data Model
This introduction will describe implementation
use cases of the CDISC ODM. It will
teach the basics of XML and expand on how that
is implemented in the operational data models.
The use cases will include exchanging data,
archival and electronic submission to
regulatory agencies such as a FDA submission. |
| Lunch
Break |
|
| 1:00 pm -
4:00 pm |
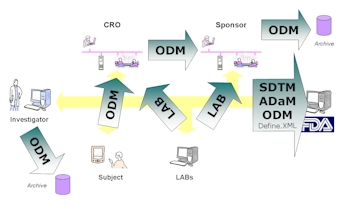
ODM Data Model
The ODM is one of the first data models
established by CDISC and has become the
standard structure which many other data
models inherit and expand upon. Other
related models include SDTM, ADaM and
DEFINE.XML. This course will give you a
foundation on the ODM and how it can be used
for data transportation between collaborators
such as CRO and also electronic submission to
regulatory organization such as the FDA.
It will deal with archival techniques and
other related metadata management to become
fully compliant.
 |
Thursday,
August 13
9:00 am - 12:00 noon |
CDISC SDTM Implementation
Implementation of the CDISC data models is no longer a theoretical academic exercise but is now entering the real world. This course will walk you through the lessons learned from implementations of CDISC SDTM version 3.1.1 and ADaM 2.0. It will cover both technical challenges along with methodologies and processes.
Some of the topics covered in this section include:
- Project Definition, Plan and Management
- Data Standard Analysis and Review
- Data Transformation Specification and Definition
- Performing Data Transformation to Standards
- Review and Validation of Transformations and Standards
- Domain Documentation for DEFINE.PDF and DEFINE.XML
The regulatory requirements are going to include CDISC in the near future and the benefits are obvious. It is therefore wise and prudent to implement with techniques and processes refined from lessons learned based on real life implementations.
CDISC standards have been in development for many years. There have been structural changes to the recommended standards going forward from version 2 to 3. It is still an evolving process but it has reached a point of critical mass that organizations are recognizing the benefits of taking the proposed standard data model out of the theoretical and putting it into real life applications. The complexity of clinical data coupled with technologies involved can make implementation of a new standard challenging. This paper will explore the pitfalls and present methodologies and technologies that would make the transformation of nonstandard data into CDISC efficient and accurate.
|
| Lunch Break |
|
| 1:00
pm - 4:00 pm |
Generating Data Definition Domain Documentation DEFINE.XML and DEFINE.PDF
This section will describe methods and tools that would enable you to document your data definition document. You can have greater understanding and management of your data if it is well
documented with data definition documentation in the format of DEFINE.PDF and
DEFINE.XML.
DEFINE.XML Implementation Examples
When you plan for a road trip, you need a map. This is analogous to understanding the data that is going to be part of an electronic submission. The reviewer requires a road map in order to understand what all the variables are and how they are derived. It is within the interest of all team members involved to have the most accurate and concise documentation pertaining to the data. This can help your team work internally while also speeding up the review process which can really make or break an electronic submission to the FDA. Some organizations perform this task at the end of the process but they really lose out on the benefits which the document provides for internal use. It is therefore recommended that you initiate this process early and therefore gain the benefit of having a road map of your data. The process that is involved in managing and creating the data definition documentation is as follows:
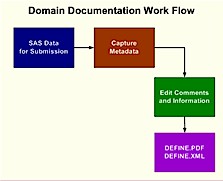
The process is an iterative one since the SAS datasets are updated. The constant need to update the documentation is therefore one of the challenges which this paper will address.
|
|